Computational & Cognitive Neuroscience
During the last decade, we have focused on detailed biologically grounded models of the cerebellum, hippocampus, and cortex which all have directly fed into the system level models tested in our research lines.
- Most notable results, together with the late John Lisman work, we have provided definite proof that the hippocampal memory system can be seen as an attractor system (Reno-Costa et al., 2015).
- We have also demonstrated that cortical gain control and contextual processing is modulated through a combination of interneurons and neuromodulators (Puigbo et al., 2018) and that stroke leads to distinct distortions of the thalamo-cortical system giving rise to new EEG based biomarkers for rapid diagnostics (van Wijngaarden et al., 2016).
- A further critical advance has been the demonstration that the “gold standard” of motor control, i.e., Feedback Error Learning, which is dominating the field for the last 40 years is incorrect and should be replaced by an active inference-based model (Maffei et al., 2017). The important consequence of this reformulation is that motor learning is now integrated into a sensori-sensory learning perspective which provides a unique unification of perceptual and behavioral learning in the integration between the cerebellum and the neocortex. Where the latter is instructing the former via anticipated or counterfactual errors rather than physical ones.
Hippocampus
Within this line of research, we have studied how the hippocampal complex computes ongoing and future sensory states.
- We have shown that the interaction between entorhinal- and hippocampal-cells are sufficient to predict future sensory streams, and, consequently, compute environmental changes.
We looked at how human hippocampal theta actively associates environmental sensory information.
- Via a biologically constrained model of border- and grid-cells we showed the computational mechanism for spatial representation error minimizations.
- We have shown that humans, like rodents, perform head-scanning behaviors for simulating the outcome of possible future spatial trajectories.

Head-orientation and decision-making duration. A Participant’s rate maps were aligned (rotated 180 deg) when necessary so that starting location would match across participants and conditions. Rate maps of low (left) and high (center) frequency conditions, as well as their difference (right) are shown. see paper for more details
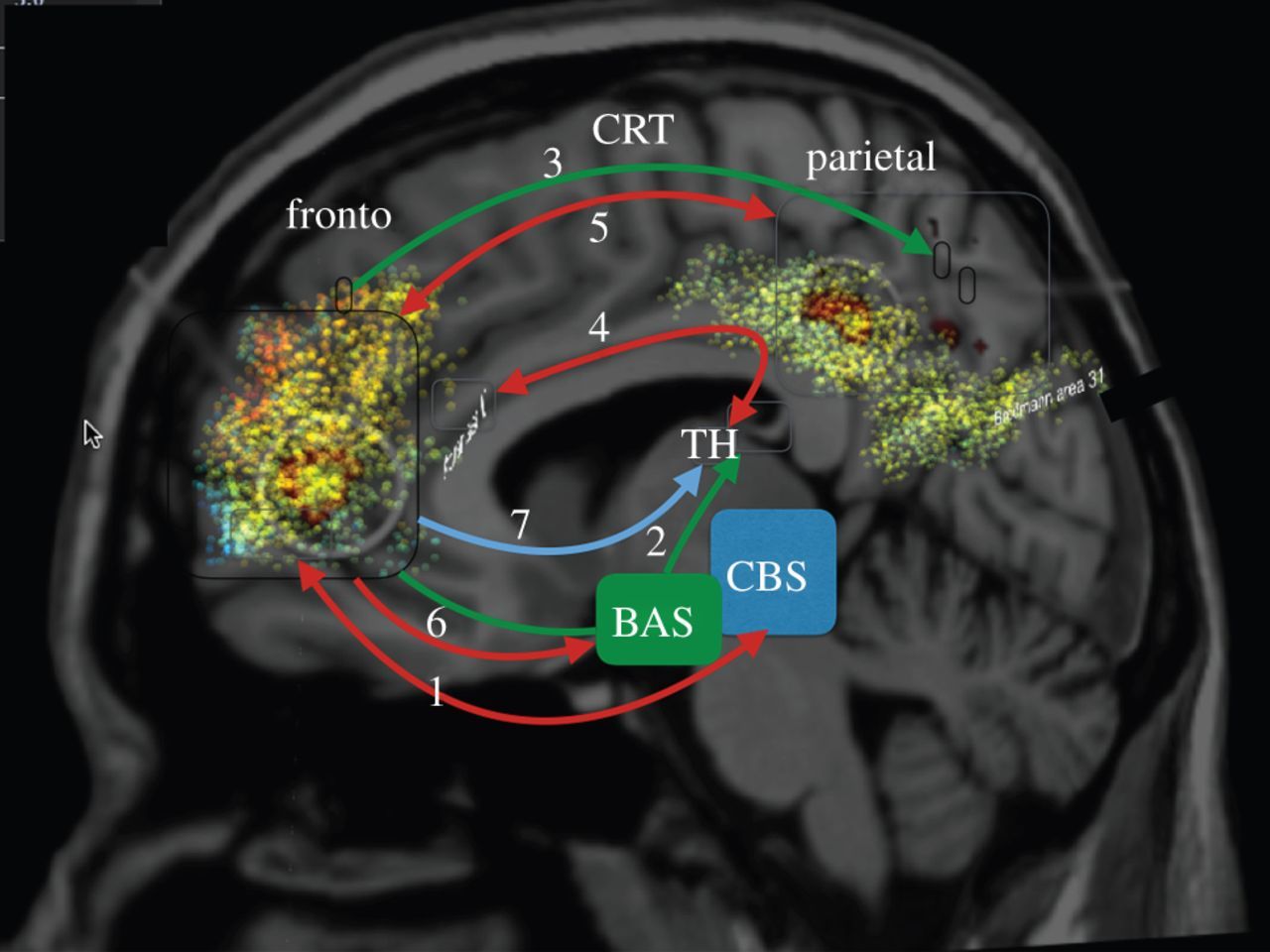
Hippocampus, Cortex and Thalamus
In this line of research, we have developed several perceptual, cognitive and motor tasks using virtual reality which were performed by epileptic patients with implanted electrodes. Recording areas varied across patients but typically included structures from the Medial Temporal Lobe (MTL, Amygdala, Entorhinal and Parahippocampal cortices and Hippocampus), Frontal and Parietal areas of which we have obtained the local field potential over > 1000 contact points. Preliminary analyses have revealed the reinstatement of stimulus-specific patterns of activity in the hippocampus during learning and testing in long-term memory, cross-frequency coupling supporting the maintenance of items in working memory, theta activity in motor areas underlying the switch from deliberative to habitual actions.
- Our findings also point to a fundamental role of phase synchronization in learning and perceiving. We specifically observed increases in Inter-Trial Coherence (ITC) for perceived versus non-perceived stimuli in subliminal tasks.
- We also found increased theta oscillations during volitional versus passive encoding of information in a spatial memory test.
- We have observed only a limited role for amplitude modulations of the LFP in recovering relevant task features. This suggests that firing rate might not be the most relevant encoding dimension when considering more advanced ecologically valid tasks in humans.
We have shown that stroke leads to distinct distortions of the thalamo-cortical system giving rise to new EEG based biomarkers for rapid diagnostics (van Wijngaarden et al., 2016)
We demonstrated that the dynamics of brain functional networks might not be governed by as many ultra-high degree hubs as a scale-free network might suggest, but show a preference for a distributed core of structural nodes that relay to the rest of the network (Zucca et al., 2016).

Cerebellum
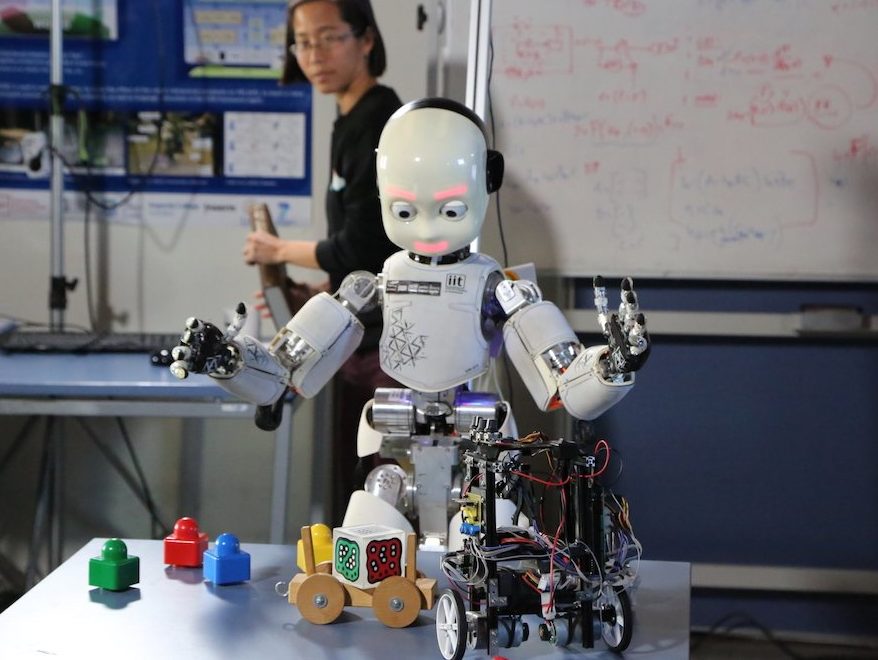
Together with developing acomputational model of the cerebellum, we have investigated whether emulating the input–output functions performed by a brain structure presents the possibility for developing neuroprosthetic systems that replace damaged neuronal circuits. With our work, we have demonstrated the feasibility of this approach by replacing the cerebellar circuit responsible for the acquisition and extinction of motor memories.
See our paper on bi-directional coupling of a prosthetic system with the central nervous system (I. Herreros et al. Front. Bioeng. Biotechnol.2014)

Biological microcircuit and synthetic counterpart. figure details here
Relevant publications
Santos-Pata D and Verschure PFMJ (2018) Human Vicarious Trial and Error Is Predictive of Spatial Navigation Performance. Front. Behav. Neurosci. 12:237. doi: 10.3389/fnbeh.2018.00237
Verschure, P.F.M.J., Zucca, R., Pacheco, D., Santos-Pata, D., Maffei, G., Puigbó, J.Y., Arsiwalla, X.D., Principe, A., Conesa, G. & Rocamora, R. The neural code of the human brain as revealed by the analysis of intracranial recordings across a range of perceptual and cognitive tasks. Society for Neuroscience 47th Annual Meeting, 2017.
Arsiwalla, X. D., Pacheco, D., Principe, A., Rocamora, R., Verschure, P., (2018). A temporal estimate of integrated information for intracranial functional connectivity Artificial Neural Networks and Machine Learning (Lecture Notes in Computer Science) 27th International Conference on Artificial Neural Networks (ICANN 2018) , Springer, Cham (Rhodes, Greece) 11140, 403-412
Santos-Pata, D., Zucca, R., and Verschure, P. F. (2016). “Navigate the unknown: implications of grid-cells “mental travel” in vicarious trial and error,” in Conference on Biomimetic and Biohybrid Systems (Cham: Springer), 251–262.
Caligiore, D., Pezzulo, G., …. and Paul F. M. J. Verschure, R. Zucca, I. Herreros (2017). Consensus paper: towards a systems-level view of cerebellar function: the interplay between cerebellum, basal ganglia, and cortex. The Cerebellum, 16(1), 203-229.
Wijngaarden, J. B. G., Zucca, R., Finnigan, S., & Verschure, P. F. M. J. (2016). The Impact of Cortical Lesions on Thalamo-Cortical Network Dynamics after Acute Ischaemic Stroke: A Combined Experimental and Theoretical Study. PLOS Computational Biology, 12(8), e1005048. https://doi.org/10.1371/journal.pcbi.1005048
Zucca, R., Arsiwalla, X. D., Le, H., Rubinov, M., & Verschure, P. F. M. J. (2016). Scaling Properties of Human Brain Functional Networks. In A. E. P. Villa, P. Masulli, & A. J. Pons Rivero (Eds.), Artificial Neural Networks and Machine Learning – ICANN 2016 (Vol. 9886, pp. 107–114). Cham: Springer International Publishing. https://doi.org/10.1007/978-3-319-44778-0_13
Maffei, Giovanni, et al. “An embodied biologically constrained model of foraging: from classical and operant conditioning to adaptive real-world behavior in DAC-X.” Neural Networks 72 (2015): 88-108.
Maffei, G., Sanchez-Fibla, M., Herreros, I., & Verschure, P. F. (2014, July). The role of a cerebellum-driven perceptual prediction within a robotic postural task. In International Conference on Simulation of Adaptive Behavior (pp. 76-87). Springer, Cham.
Herreros, I., Giovannucci, A., Taub, A. H., Hogri, R., Magal, A., Bamford, S., … & Verschure, P. F. (2014). A cerebellar neuroprosthetic system: computational architecture and in vivo test. Frontiers in bioengineering and biotechnology, 2, 14.
Brandi, S., Herreros, I., & Verschure, P. F. (2014, July). Optimization of the anticipatory reflexes of a computational model of the cerebellum. In Conference on Biomimetic and Biohybrid Systems (pp. 11-22). Springer, Cham.
Herreros, I., Maffei, G., Brandi, S., Sánchez-Fibla, M., & Verschure, P. F. (2013, November). Speed generalization capabilities of a cerebellar model on a rapid navigation task. In 2013 IEEE/RSJ International Conference on Intelligent Robots and Systems (pp. 363-368). IEEE.
Rennó-Costa, César, John E. Lisman, and Paul FMJ Verschure. “The mechanism of rate remapping in the dentate gyrus.” Neuron 68.6 (2010): 1051-1058.
Guanella, Alexis, Daniel Kiper, and Paul Verschure. “A model of grid cells based on a twisted torus topology.” International journal of neural systems 17.04 (2007): 231-240.
Wyss, Reto, Peter König, and Paul FM J. Verschure. “A model of the ventral visual system based on temporal stability and local memory.” PLoS biology 4.5 (2006): e120.
Hofstötter, C., Gil, M., Eng, K., Indiveri, G., Mintz, M., Kramer, J., & Verschure, P. F. (2005). The cerebellum chip: an analog VLSI implementation of a cerebellar model of classical conditioning. In Advances in neural information processing systems (pp. 577-584)
HofstoÈtter, C., Mintz, M., & Verschure, P. F. (2002). The cerebellum in action: a simulation and robotics study. European Journal of Neuroscience, 16(7), 1361-1376
Verschure, P. F., & Mintz, M. (2001). A real-time model of the cerebellar circuitry underlying classical conditioning: A combined simulation and robotics study. Neurocomputing, 38, 1019-1024.